Corrosion of concrete: types, mechanisms, methods of
What is it - corrosion of concrete and reinforced concrete? Why do corrosion processes occur in reinforced concrete structures? What ways can prevent their development? In the article we will try to answer these questions.

What it is
Corrosion of concrete - the process of loss of strength or destruction of concrete and reinforced concrete structures associated with aggressive environmental exposure. It seems that the reader does not need to explain how corrosion of metal structures proceeds. In general, the same thing happens with concrete: over time, it is partially transformed into other materials with completely different mechanical properties.
Let's clarify: ferro-concrete structures, of course, also suffer from ordinary rust. In most cases, the reinforcement does not have high corrosion resistance.

Types and mechanisms
Помните пословицу «где тонко, там и рвется»? Она в полной мере относится к деградации любых конструкционных материалов.
Reinforced concrete is a composite of several types of raw materials that differ in mechanical strength and resistance to different types of external influences.
| Material | Properties |
| Sand | Quartz crystals are exceptionally chemically stable, do not degrade over time. |
| Rubble | As a fill, rock gravel is usually used, its chemical and mechanical properties differing little from quartz sand. Concentrated alkalis and acids can affect its strength. |
| Fittings | The contact of steel in water and air (and concrete, as we remember, is vapor-permeable) always gives a very predictable result. Even under a protective layer of concrete, the reinforcement will gradually rust. The release of reinforcement to the surface due to the destruction of the structure will speed up the process many times. |
| Cement stone | Binder - cement - after setting turns into a relatively strong, but not characterized by chemical inertness cement stone. One of its main components - hydrated lime Ca (OH) 2 - is easily dissolved by water and reacts with other chemicals. It is with the destruction of the cement stone that the corrosion process usually begins. |
Let's analyze the main types of corrosion and the mechanisms of their occurrence.
erosion
Despite its high density, concrete is a porous material. The reason is that the setting of the cement and the subsequent drying of the mortar are accompanied by a significant decrease in its volume.
Pay attention: porous gas and foam concrete is a separate conversation. In their case, the pores are created intentionally - by introducing into the solution foam or gas-forming components (as a rule, aluminum powder). The goal is to give concrete maximum insulating qualities.
Moistening the concrete with subsequent uneven evaporation of water will lead to the gradual movement of water through the pores. In the process of moving, that same slaked lime Ca (OH) 2 will gradually leach out; Well, since the binder in the thickness of the concrete becomes smaller, its strength decreases.
The most vividly the process of leaching is exhibited by efflorescence — white stains and growths on the surface of the concrete, remaining where it often gets wet. Their presence suggests that the design is rapidly losing strength.

Acid decomposition
Under the influence of acids and their aqueous solutions in concrete can occur a lot of destructive processes.
Let us analyze the most simple.
- When exposed to acids, slaked lime combines with atmospheric carbon dioxide to form insoluble salt and water.. The formula describing the reaction is Ca (OH) 2 + CO2 = CaCO3 + H2O.
It would seem - why grieve if the soluble calcium compound is replaced by a more stable one? After all, the process of leaching in this case should completely stop. Not here it was: CaCO3 crystals do not just fill the pores - they seek to expand, crack them; as a result, the concrete begins to crack.
- With an excess of water (in simple terms, in wet concrete), further transformation of minerals takes the form CaCO3 + CO2 + H2O = Ca (HCO3) 2. Полученный бикарбонат кальция снова растворим для воды; более того - слишком растворим: он стремительно вымывается, оставляя после себя поры и… падение конструкционной прочности.
- In the presence of a solution of hydrochloric acid, slaked lime is converted to calcium chloride: Ca (OH) 2 + 2HCl = CaCl2 + 2H2O. And this salt is extremely soluble in water; the result is quite predictable - again, the weakening of the structure.
Sulphate decomposition
In the conditions of the chemical industry (in particular, producing fertilizers), the so-called sulfate corrosion of concrete is quite common.

As a result of interaction with slaked lime sulfates and aluminates present in the cement, ettringite hydrosulfoaluminate (3CaO • Al2O3 • 3CaSO4 • 32H2O) is formed. The crystals in the process of growth cause significant stresses, significantly exceeding the strength characteristics of the cement stone.
Rusting reinforcement
Здесь все просто и понятно: контакт низкоуглеродистых сталей с водой и воздухом приводит к образованию малопрочного Fe2O3 и более сложных окислов и солей. Армирование должно воспринимать нагрузки на растяжение; при падении прочности арматуры существенные нагрузки на изгиб приводят к появлению трещин и… ускоренному падению прочности уцелевшего армирования вследствие прямого контакта с водой и воздухом (см.также статью «Подпорные стены из бетона: технология возведения от профессионалов»).
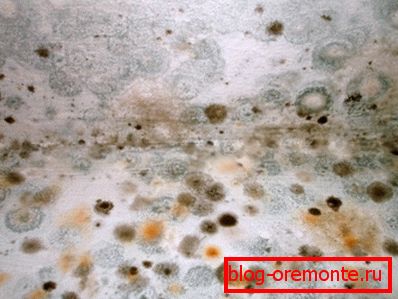
Biodegradation
The consequences of high humidity at temperatures above zero are well known: structures made of brick, stone and concrete are settled with moss and mold.
As a result, destruction proceeds in two ways:
- The notorious lime and its compounds serve as food for the fungus.
- The accumulation of metabolic products in the pores leads to an increase in internal stresses.
Frosty destruction
Imagine what happens to a wet concrete site when the temperature drops below zero.
- The water in its pores begins to crystallize.
- Ice, which has a larger volume than water, tends to expand the pores. Microcracks appear in the structure; as they are expanded, the corrosion of reinforcement is connected to the destruction of reinforced concrete.
Ways to protect
So, the mechanisms of destruction we have studied. Is it possible to protect concrete and reinforced concrete structures from corrosion? Can appropriate measures be taken at home with their own hands?
Strategy
First, find out what ways we have to go.
| The complex of measures | Explanations |
| Valve protection | Increasing the corrosion resistance of the reinforcing cage will prevent it from rusting inside the concrete and upon reaching the surface. |
| Sealing chemical additives | As a rule, they reduce the number of pores or make the pores closed. As a result, the permeability of the material to water and air decreases, less frequently, unstable slaked lime is replaced by more chemically resistant compounds. |
| Filling the pores | The finished concrete structure can be modified by penetrating impregnations injected through holes drilled in it or simply deposited on the surface. |
| Surface protection | This includes all sorts of waterproofing measures (roll and coating). Painting with paintwork materials falls into this category. |
| Bioprotection | Antiseptic impregnations nullify the biodegradation, killing the mold itself, its spores and preventing their reappearance. |

Tactics
And now let's make a bit more concrete the list of possible measures, having described some of them.
Industrial conditions
As protection of reinforced concrete structures against corrosion is carried out in conditions of industrial enterprises, multi-family construction, etc. - simply put, when is it possible to use complex technologies that require special equipment?
We mention a few frequently used solutions.
- Cementing. Через пробуренные в толще конструкции отверстия под давлением нагнетается цементное молочко, приготовленное в пропорции 1:10 (цемент-вода), с небольшой (не более 7% от массы цемента) добавкой хлористого кальция. Filling the pores способствует увеличению плотности бетона и уменьшению количества открытых пор в нем.
- Silicization is reduced to the successive injection of sodium liquid glass and calcium chloride. In the process of processing the pores are filled with a mixture of poorly soluble calcium silicate and insoluble silica.

- Bitumenisation - the process of filling the pores with bitumen at a temperature of 200-220C. The method is extremely effective, but can only be carried out with a minimum moisture content of the structure.
Useful: the main problem in drilling holes for injection of solutions is not to cause an increase in internal stresses in the thickness of the structure. From this point of view, diamond drilling in holes in concrete is optimal: it does not create shock loads and does not cause chipping of the edges of the hole.
For the opening and dismantling of structural elements, cutting of reinforced concrete with diamond circles is used: they have a much larger resource compared to abrasive circles for stone and, most importantly, rebar is perfectly cut.
Home conditions
Of course, the protection of concrete against corrosion is possible without the use of high-tech equipment.
- Protective paint - the simplest and most obvious solution. In particular, we can recommend the so-called rubber water-dispersed dyes: they reliably waterproof the surface of the concrete with minimal time and effort. The price of a kilogram of rubber paint starts from about 130 rubles.

- Processing with liquid glass is also able to protect the concrete from destruction. Instructions for its use is extremely simple: sodium liquid glass is diluted with water 1: 1 and applied to the concrete surface with a brush or roller in 2-3 layers without intermediate drying.
- The most effective solution is penetrating waterproofing impregnations (Penetron and its analogues). They are applied on wet concrete and penetrate to a depth of one meter. Penetron causes the crystallization of calcium compounds that completely fill the pores.
- At the stage of concrete preparation, various reinforcing additives can be introduced into it. Here are the names of several domestic drugs: Milonafta, SDB (sulfite yeast brew), NGL-94 (silicone fluid).

Conclusion
Разумеется, в рамках небольшой статьи нами затронуто лишь несколько из длинного перечня возможных решений (читайте также статью «Бетонные безнапорные трубы: нормативные документы, применение, альтернативы»).
The video in this article will offer the reader more information about how concrete corrosion manifests and how it can be defeated. Successes!